By Sophia Friesen, Ph.D., Manager, Research Communications, University of Utah Health
Why it matters: Federally funded basic research at the University of Utah laid the groundwork for a new HIV-prevention drug now approved for clinical use—demonstrating how curiosity-driven science delivers real-world impact.

Wes Sundquist wasn’t trying to become one of TIME’s 100 most influential people in the world. He wasn’t shooting for Science Magazine’s Breakthrough of the Year or the Horwitz prize, considered one of the strongest predictors of a Nobel. He didn’t know his discoveries would lay the foundations for a drug that’s poised to change the course of the HIV epidemic nationally and worldwide. Those accomplishments were rooted not in a desire for fame and glory, but from interest in the natural world.
Driven by curiosity
“We’re driven by curiosity to discover things that we don’t understand,” says Sundquist, who is a distinguished professor and department chair of biochemistry. “It’s not so different from other kinds of adventures. The same thing that drives people to climb mountains drives us to discover how molecular machines work.”
The Sundquist lab does basic research on the HIV virus—not basic in the sense that it’s simple, but basic in that it’s driven by a desire to answer fundamental questions about biology.
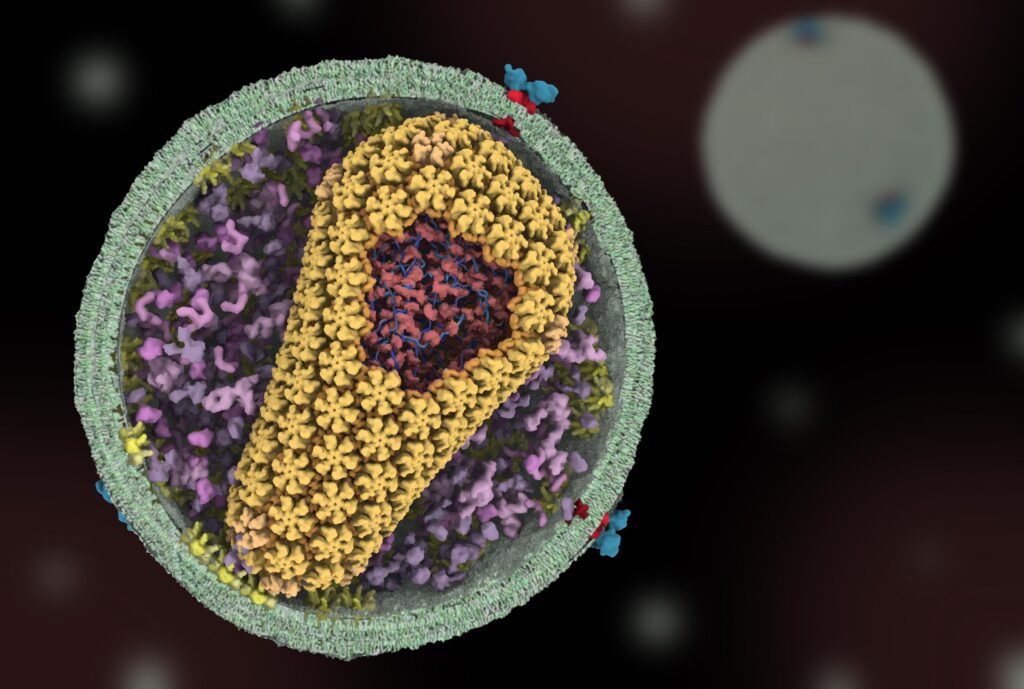
Two decades ago, one question that drove the lab was: how does the virus assemble into its characteristic cone shape? As they explored the virus’s microscopic architecture, the lab found that one of its key elements—a protein that forms a shell around the virus’s genetic material—was extremely sensitive to change. Small changes to many parts of this protein, called the capsid protein, significantly disrupted the virus’s ability to infect cells.
From discovery to drug
It raised the question of whether a drug that targeted the capsid could also stop infection, prompting American pharmaceutical company Gilead Sciences to use Sundquist’s research to develop a drug called lenacapavir that targeted the capsid, with Sundquist as a consultant.
Clinical trials of lenacapavir were “spectacularly successful”, Sundquist says. A single injection of the drug prevents HIV infection with close to 100% efficacy for six months. “Lenacapavir almost completely prevents the transmission of HIV into at-risk populations. This is just an amazing result.”
In June 2025, lenacapavir received FDA approval for use as HIV prevention. Every year, 31,000 people in the US and 1.3 million people worldwide contract HIV. Widespread distribution of the drug could dramatically lower those numbers, saving lives and reducing treatment costs.
The power of basic research
Academic labs like Sundquist’s are critical to drug development, says Tomas Cihlar, PhD, senior vice president of virology research at Gilead. “Without investing in basic research, we in industry would not have the foundational information about biological functions and systems.”
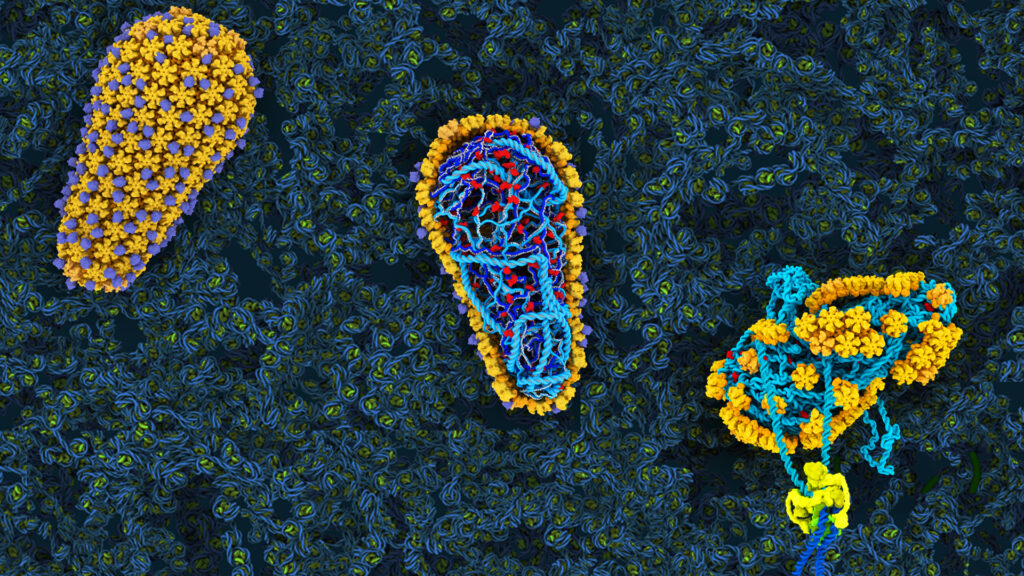
Lenacapavir is a testament to the power of basic research, even when it’s not obvious how scientific findings might be useful. 20 years ago, the precise microscopic architecture of HIV was an academic problem, a question driven by the beauty of molecular machines. But the knowledge that research uncovered enabled the development of a drug that could change the course of a global epidemic.
Today, basic research continues to plant the seeds for industry collaboration and real-world solutions. Sundquist’s lab is uncovering features of the ESCRT pathway, a fundamental process that helps sort molecules inside cells, that suggest targets for broad-acting anticancer therapies. Other work builds a vital knowledge base to keep Utah and the nation prepared for what’s next.
“We don’t know what we don’t know,” says Chris Hill, distinguished professor of biochemistry, “but we do know there’s a lot of it. Even if you don’t quite know how it might become useful, you’ve got to find it out, because some of it will be transformative.”

